FDA Regulations 1.20
Free Version
Publisher Description
FDA Regulations - The full text of FDA Food, Medical Device and Drug GMP regulations
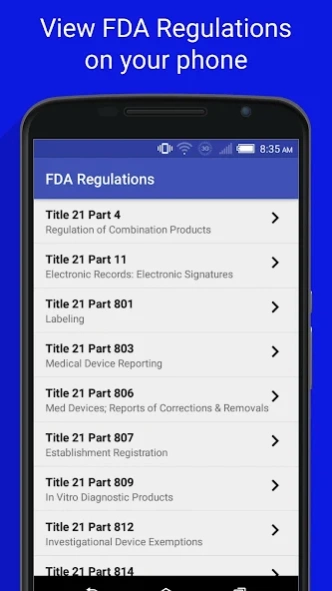
This app contains the full content of the United States Food and Drug Administration (FDA) Food, Medical Device and Drug GMP Regulations. The following Title 21 Parts are included:
21CFR4: Regulation of Combination Products
21CFR11: Electronic Records: Electronic Signatures
21CFR108: Emergency Permit Control
21CFR110: Current Good Manufacturing Practice in Manufacturing, Packing or Holding Human Food
21CFR111: Current Good Manufacturing Practice in Manufacturing, Processing, Packing or Holding Operations for Dietary Supplements
21CFR113: Thermally Processed Low-Acid Foods Packaged in Hermetically Sealed Containers
21CFR114: Acidified Foods
21CFR117: Current Good Manufacturing Practice, Hazard Analysis, and Risk-Based Preventive Controls for Human Food
21CFR120: Hazard Analysis and Critical Control Point (HACCP) Systems
21CFR123: Fish and Fishery Products
21CFR210: Current Good Manufacturing Practice in Manufacturing, Processing, Packing, or Holding of Drugs; General
21CFR211: Current Good Manufacturing Practice for Finished Pharmaceuticals
21CFR801: Labeling
21CFR803: Medical Device Reporting
21CFR806: Med Devices; Reports of Corrections & Removals
21CFR807: Establishment Registration
21CFR809: In Vitro Diagnostic Products
21CFR812: Investigational Device Exemptions
21CFR814: Premarket Approval of Medical Devices
21CFR820: Quality System Regulation
21CFR821: Medical Device Tracking Requirements
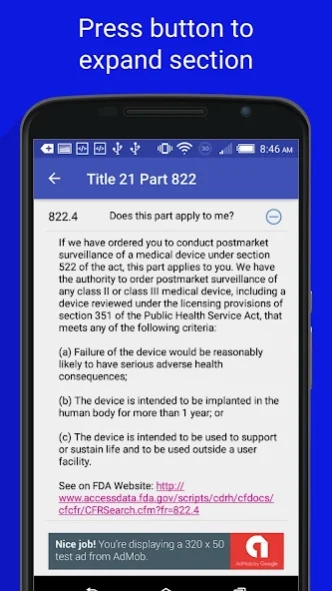
21CFR822: Postmarket Surveillance
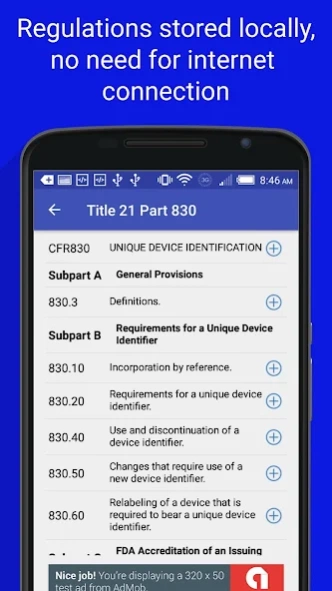
21CFR830: Unique Device Identification
21CFR860: Classification Procedures
The FDA Food, Drug, and Medical Device regulations are split up by section, click on a section to expand that specific section. Click again to hide. Each section also contains a link to the FDA website which contains the regulation so you can verify that it is up to date.
The FDA regulations are stored on your device and don't require an internet connection to access.
This app is a useful for auditors and as a general reference, just keep your phone handy instead of carrying around a reference book.
About FDA Regulations
FDA Regulations is a free app for Android published in the Office Suites & Tools list of apps, part of Business.
The company that develops FDA Regulations is Jacob Kearns. The latest version released by its developer is 1.20.
To install FDA Regulations on your Android device, just click the green Continue To App button above to start the installation process. The app is listed on our website since 2022-10-18 and was downloaded 1 times. We have already checked if the download link is safe, however for your own protection we recommend that you scan the downloaded app with your antivirus. Your antivirus may detect the FDA Regulations as malware as malware if the download link to com.jacobkearns.fdaregulations is broken.
How to install FDA Regulations on your Android device:
- Click on the Continue To App button on our website. This will redirect you to Google Play.
- Once the FDA Regulations is shown in the Google Play listing of your Android device, you can start its download and installation. Tap on the Install button located below the search bar and to the right of the app icon.
- A pop-up window with the permissions required by FDA Regulations will be shown. Click on Accept to continue the process.
- FDA Regulations will be downloaded onto your device, displaying a progress. Once the download completes, the installation will start and you'll get a notification after the installation is finished.